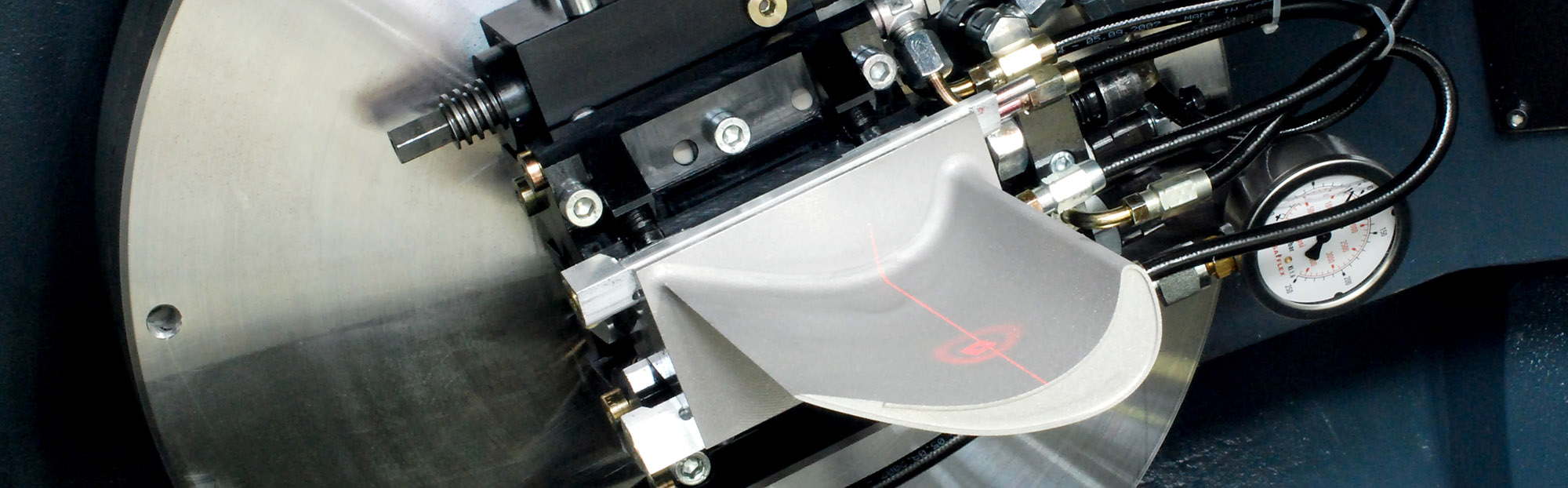
The basis of any competitive production is state-of-the-art manufacturing processes. Only those who continuously optimize their performance, act flexibly at the same time and are able to comply with the high demands on quality assurance will continue to be successful on the market in the future. In particular, the constant flexibilization of the production process and increased demands on quality assurance present companies with new challenges. Measuring systems that support these requirements are therefore essential for the production of the future.
Fraunhofer IPT is therefore working on making production processes more efficient through the use of measurement technology, increasing productivity and guaranteeing high quality. Measurement technology is thus a key technology for making complex production processes possible in the first place. In particular, the production integration of metrological systems offers a technological advantage.
The goal of production-integrated quality assurance is always the recording and evaluation of relevant quality parameters, if possible in spatial and temporal proximity to the actual production process. Actual values recorded in this way during the production cycle can be quickly processed and made available to the process again as setpoint values and used for control purposes. This allows complex production processes to be better controlled and individually adapted to the product.
Production and process-integrated measurement systems, intelligent measurement and control strategies, and appropriate information technology integration into the production environment accelerate response times to process changes and target deviations, thus enabling networked, adaptive production.